Aggregation
Reduce the Risk of Immunogenicity
Understanding the mechanism of aggregation leads to effective risk management for aggregates within the entire development life cycle as the therapeutic protein is subjected to both upstream and downstream processes.
Aggregation
AGGREGATION
Protein aggregation is a concern in the manufacturability of therapeutic proteins and careful control during upstream and downstream processes is important to mitigate the formation of aggregates. Monitoring aggregation is an important aspect in quality control of biopharmaceuticals, especially given aggregates have been associated with unwanted immune response.
Aggregation is also an important consideration in formulation optimization. Historical review of the data can also be deployed over the product lifetime to assess the impact of any supply chain or process modifications.

AGGREGATE IDENTITY
Determining the difference between a protein aggregate and a non-proteinaceous particulate is a first step in aggregation analysis. From that point, identifying the pathways that lead to the formation of aggregates is an important process for understanding how to mitigate aggregation.
ProteinMentor is uniquely able to both differentiate particulates from aggregates and to identify the process leading to aggregation via its different mechanisms such as the formation of charge variants, denaturation or self-association due to formulation conditions or physical stresses that decrease the stability of the therapeutic protein.
AGGREGATE SIZE
Quantification of aggregation according to size is an important aspect of aggregation characterization. Protein aggregation has been identified in a wide size range, however the µm – mm range has been associated with the potential for immunogenicity.
ProteinMentor monitors aggregates within the immunogenicity size range for a given drug substance, sample, or drug product. Visual inspection of the images can show features between 4.3 µm and 2 mm but even without visual evidence, the underlying spectral data can assess changes leading to the formation of potentially dangerous aggregates before they occur.
The number, position, and size of the particles within each sample can be monitored to build a detailed picture of the overall changes in stability and their potential impact on safety and efficacy.
EXTENT OF
AGGREGATION
The extent of aggregation can be defined in two ways. One is in determining the region of the protein that is prone to aggregation. The other is to evaluate whether there is a significant presence of aggregates in the solution. Both are valuable factors to manage the risk of aggregation.
Our patented method allows for the quantitative assessment of the:
(1) amount of biotherapeutic that is aggregated
(2) identify the region prone to aggregation.
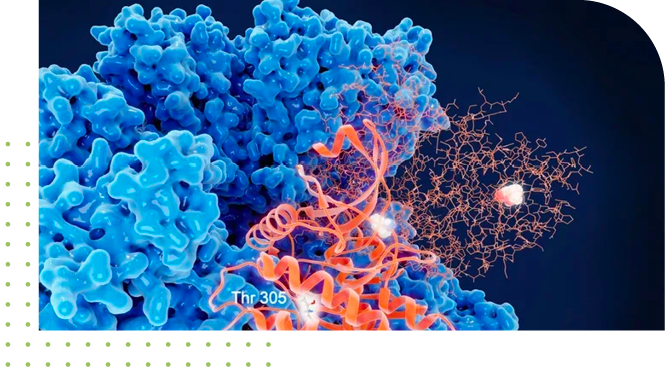
MECHANISM OF
AGGREGATION
Mechanisms of aggregate and particulate formation are complex and may include:
(1) post-translational modifications;
(2) exposure to stressors that lead to oxidation, denaturation or deamidation, or
(3) formulations with less-than-optimal conditions for solvent accessible regions of the protein, which can result in self-association or even adsorption events.
Molecular changes that occur as the protein sample is stressed can be determined using well-established spectral correlational algorithms post HS image acquisition. Less stable regions of the protein can be assessed through comparative analysis. The mechanism of aggregation is thereby understood in much more detail.
REGIONS PRONE TO AGGREGATION
ProteinMentor provides both spatial and temporal resolution at the amino acid level. The high selectivity and sensitivity of the technique in combination with the dedicated software applications allows for the direct determination of the region prone to aggregation/self-association. This is achieved by the monitoring of a unique signature peak associated with aggregation, while assessing the conformational changes and the weak interactions that govern the aggregation process. This information is crucial for de-risking the therapeutic protein candidate through re-engineering or formulation optimization.

Aggregation
Application Note
This Application Note illustrates the use of the ProteinMentor platform for a comparative assessment of the stability of an array of proteins in solution. The unique combination of microscopy and spectroscopy, along with the ability to apply a thermal stress across the array of samples, provides for real-time stability assessment in a single experiment. Contact our team to learn more about an evaluation of our platform.
